


To investigate the immune response and mechanisms associated with severe coronavirus disease 2019 (COVID-19), we per-formed single-cell RNA sequencing on nasopharyngeal and bronchial samples from 19 clinically well-characterized patients with moderate or critical disease and from five healthy controls. We identified airway epithelial cell types and states vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In patients with COVID-19, epithelial cells showed an average three-fold increase in expression of the SARS-CoV-2 entry receptor ACE2, which correlated with interferon signals by immune cells. Compared to moderate cases, critical cases exhibited stronger interactions between epithelial and immune cells, as indicated by ligand–receptor expression profiles, and activated immune cells, including inflammatory macrophages expressing CCL2, CCL3, CCL20, CXCL1, CXCL3, CXCL10, IL8, IL1B and TNF. The transcriptional differences in critical cases com-pared to moderate cases likely contribute to clinical observations of heightened inflammatory tissue damage, lung injury and respiratory failure. Our data suggest that pharmacologic inhibition of the CCR1 and/or CCR5 pathways might suppress immune hyperactivation in critical COVID-19.
Read the full manuscript, published in Nature Biotechnology, and the BIH press release.
 Copyright Shutterstock/Irina Shi
Copyright Shutterstock/Irina Shi
The current globally emergent SARS-CoV-2 infection wave with high human-to-human transmission rates affecting the human respiratory system severely challenges public health. There is an urgent demand for increasing our understanding of the COVID-19 pathogenesis, especially host factors and mechanisms facilitating the infection and replication of the virus in the human respiratory tract. Similar to SARS-CoV, responsible for the global SARS outbreak in 2002-2003, recent data suggest that also SARS-CoV-2 is able to enter the host cells via binding to ACE2 and priming of its S protein by the serine protease TMPRSS2. Although it was shown previously that ACE2 is expressed on low levels in cells of the respiratory tract, detailed analysis of the cell types co-expressing both the receptor and the proteases involved in the priming process is still missing. Here, we investigate ACE2 and TMPRSS2 expression levels and their distribution across cell types in lung tissue (twelve donors, 39,778 cells) and in cells derived from subsegmental bronchial branches (four donors, 17,521 cells) by single nuclei and single cell RNA sequencing, respectively. While TMPRSS2 is expressed in both tissues, in the subsegmental bronchial branches ACE2 is predominantly expressed in a transient secretory cell type. Interestingly, these transiently differentiating cells show an enrichment for pathways related to RHO GTPase function and viral processes suggesting increased vulnerability for SARS-CoV-2 infection. Our data provide a rich resource for future investigations of COVID-19 infection and pathogenesis.
Read the full manuscript, published in EMBO Journal
Find the read count data at figshare and Mendeley.
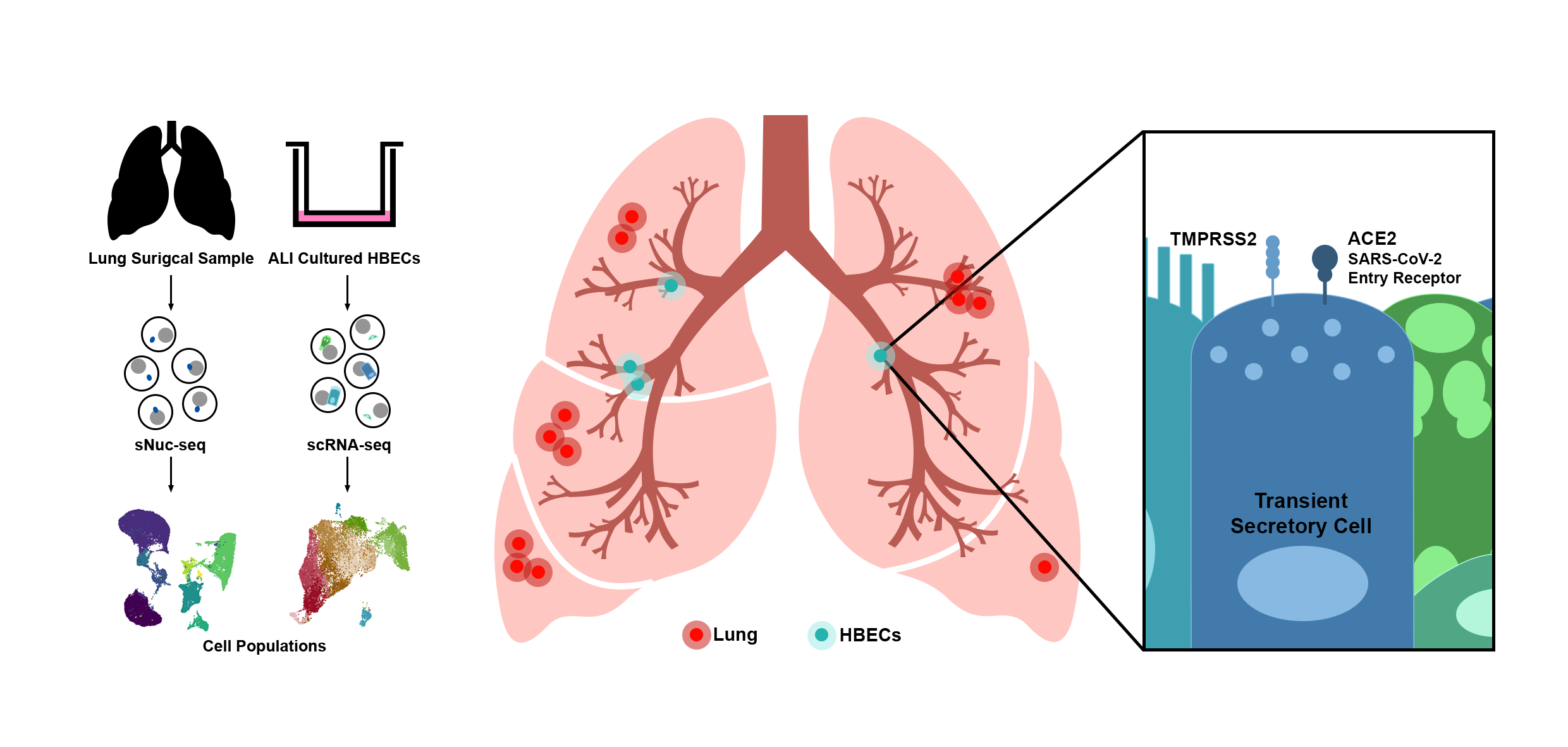 Overview figure Lukassen et al. (2020): SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J
Overview figure Lukassen et al. (2020): SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J
With the MapMyCorona webite, we provide the possibility to compute similarities of any sequence (protein or nucleotide) to already available viral SARS-CoV-2 sequences. The website displays the matching hits on a world map, together with temporal information, and offers various display options. Hence, using this website, one can follow the geographical and temporal distribution of SARS-CoV-2 sequences, and track the spread of viral strains.
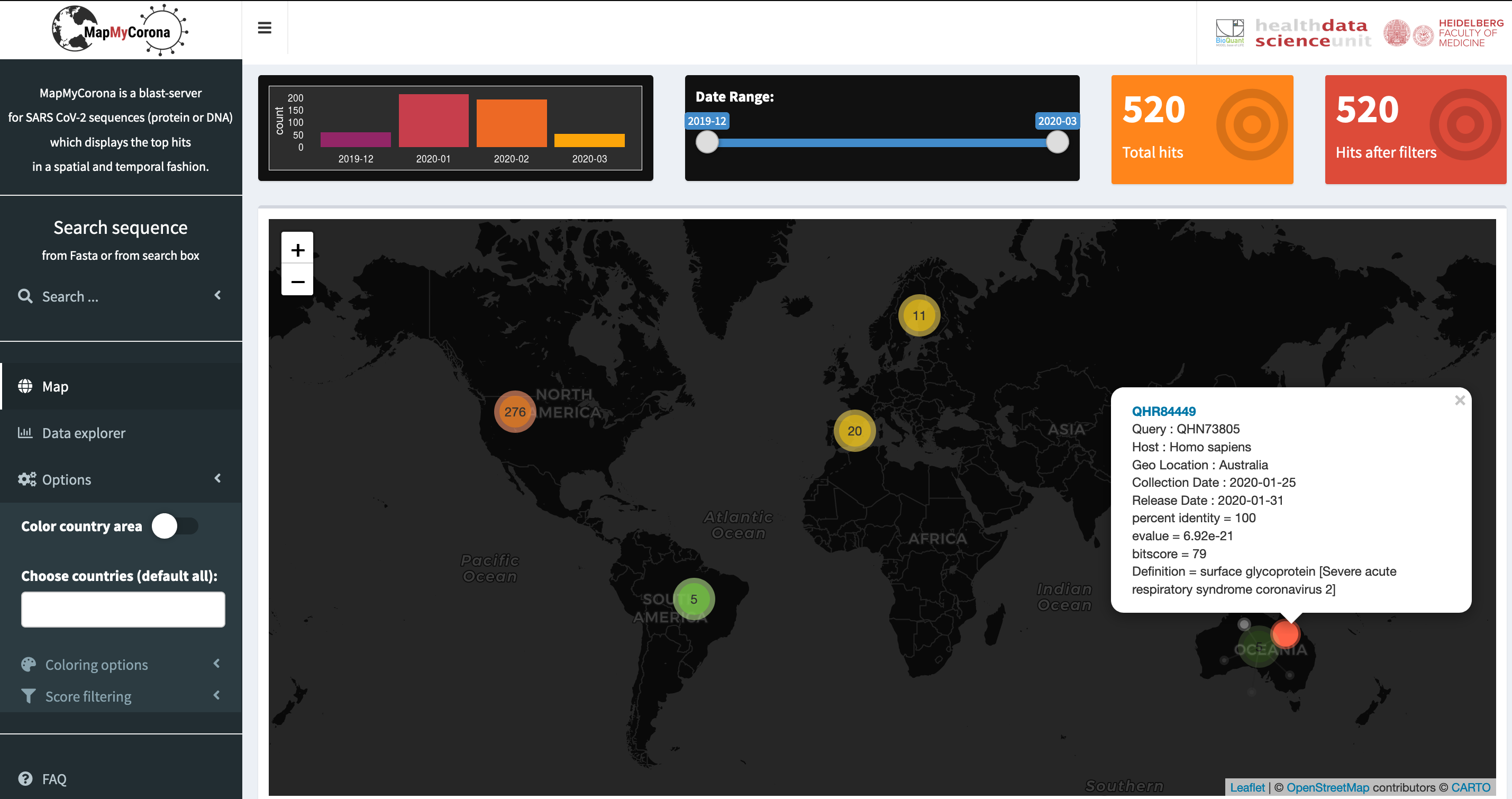
Screenshot of the MapMyCorona website displaying the geographical distribution of similar sequences to query SARS-CoV-2 sequence. Details of the matching sequences can be obtained by clicking on the hits.
Magellan: COVID-19 Omics Explorer
Magellan is a web application for the display and analysis of next-generation sequencing data with a focus on COVID-19 diseases. The application runs on the de.NBI cloud infrastructure. The focus of the omics data is on single-cell sequencing data, but other omics data, such as epigenetic data, will also be integrated in the long term. The visualization of bulk Whole Genome Bisulfite Sequencing (WGBS) Data will allow an analysis of condition dependent methylation patterns in promotor regions of coding genes and certain enhancer regions. The average CpG methylation of a genetic region is used as a marker for the specific Region.
The goal is to provide free access to the highly relevant data of COVID-19 patients for the community. The data can be freely accessed via customizable plots. This enables scientists and medical professionals to test their own hypotheses or generate new ones. If required, extended direct access to the data can be requested and checked. Magellan currently provides access to previously published data on gene expression in various lung tissue samples (see BioRxiv preprint). Magellan will soon provide access to data on the temporal gene expression of COVID-19 patients.
See also de.NBI webite for further COVID-19 related resources.
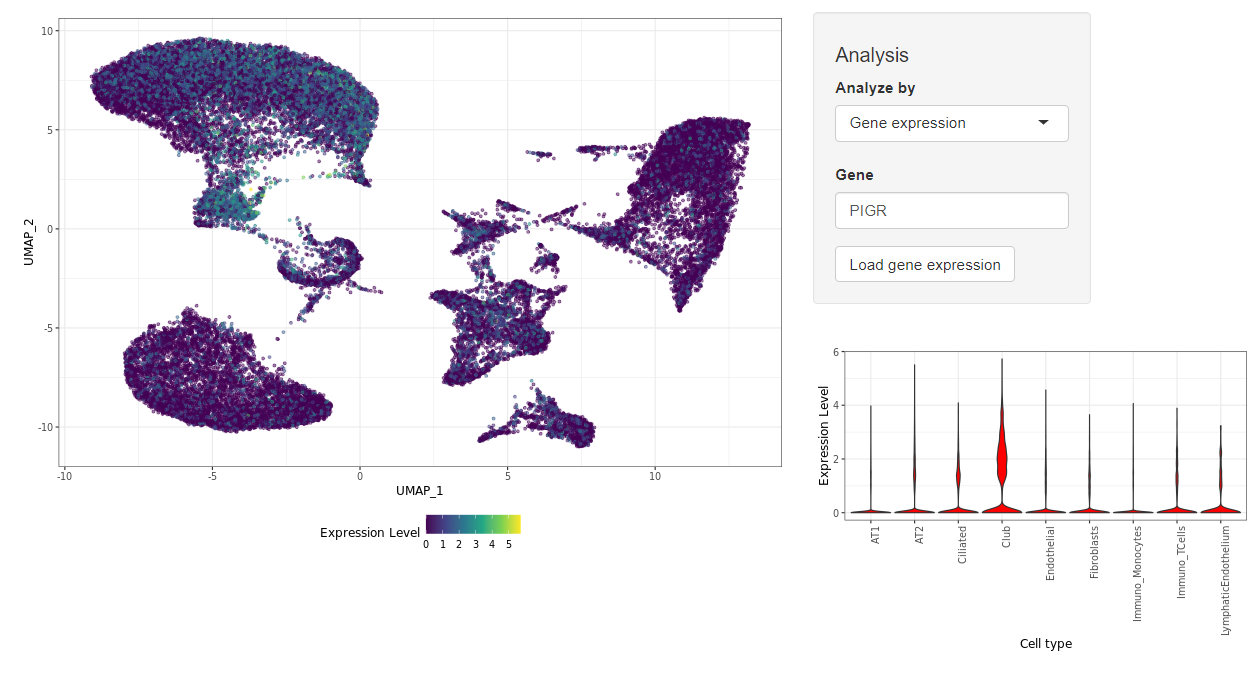
Screenshot of Magellan
Stay tuned. Further data and results will be continuously added here .

Roland Eils is the founding director of the Center for Digital Health at Berlin Institute of Health (Charité, Berlin) and the Health Data Science unit at the Medical Faculty of Heidelberg University. Before, he was founding and managing director of Heidelberg University’s Systems Biology Center BioQuant and coordinator of the cancer genomics program at the German Cancer Research Center. His group has delivered significant contributions to the field of systems biology and cancer genomics and is pioneering the field of digital health in research and care.
Prof. Dr. Roland Eils
Founding Director of the BIH@Charité - Center for Digital Health
Our colleagues from Northestern University in Boston are organising the 18th INTERNATIONAL CONFERENCE ON SYSTEMS BIOLOGY OF HUMAN DISEASE – SBHD 2026 in Boston from June 08-10. This years Conference

The Berlin Institute of Health at Charité (BIH) coordinates the pan-European research initiative "EU-CiP" (European Cancer Information Portal). Supported by 12 million euros from the EU’s Horizon

The Digital Health Center once again organises the INTERNATIONAL CONFERENCE ON SYSTEMS BIOLOGY OF HUMAN DISEASE – SBHD 2025 in Berlin from June 16-18. Don’t miss this opportunity to participate in a

Our colleagues at Vanderbilt University organise the 16th INTERNATIONAL CONFERENCE ON SYSTEMS BIOLOGY OF HUMAN DISEASE – SBHD 2024 this year from June 10-12. Don’t miss the opportunity to participate
The Hub for Innovations in Digital Health (HiDiH) brings together two independent sites of excellent research and development in Berlin and in Heidelberg. HIDIH’s major branch in Berlin is the Center for Digital Health at the Charité and the Berlin Institute of Health (BIH).
If we caught your attention, you are interested in our work and would like to get in touch with us, please contact us via franziska.mueller@bih-charite.de



